Double outlet right ventricle (DORV) is a complex congenital cardiac malformation, in which both the pulmonary artery and aorta arise predominantly from the right ventricle.
Double outlet right ventricle (DORV)
Abstract: Double outlet right ventricle (DORV) is a complex congenital cardiac malformation, in which both the pulmonary artery and aorta arise predominantly from the right ventricle. This anomaly is usually associated with a ventricular septal defect (VSD), and a degree of outflow tract obstruction. Extracardiac malformations and genetic anomalies can be associated as well. Prenatal diagnosis is important as neonatal intervention is often required.
Keywords: Double-outlet right ventricle, congenital heart defect, fetal echocardiography
Author: Inbal Willner1, Jane Lougheed2
- Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, The Ottawa Hospital, Ottawa, Ontario, Canada
- Department of Pediatrics, Division of Cardiology, The Children’s Hospital of Eastern Ontario (CHEO), Ottawa, Ontario, Canada
Reviewer: Karen Fung-Kee-Fung
View the Patient Information leaflet
Definition
Double-outlet right ventricle (DORV) is a complex congenital heart disease, wherein both the pulmonary artery and aorta are committed either >50% or completely to the morphological right ventricle (RV). DORV encompasses a wide spectrum of cardiac malformations which differ with regard to the variable spatial relationship of the great arteries, the location of a ventricular septal defect (VSD), and the presence or absence of pulmonary and less commonly, aortic outflow obstruction 1.
The VSD in DORV is described by the location of the VSD relative to the outflow tracts. There are 4 typical locations of VSD described:
• Sub-aortic type (most common)
• Sub-pulmonary type
• Double committed (both subaortic and sub-pulmonary position) type
• Remote type (nonrelated to the outflow tracts)
The anatomic relationship of the great arteries in DORV is variable and includes:
• Aorta located posterior and rightward of pulmonary artery (PA).
• Aorta located to the right/side-by-side with PA.
• Aorta located to the right and anterior of PA.
• Aorta located to the left and anterior of PA.
The classification of DORV is primarily based on the location of VSD and the presence/absence of pulmonary stenosis (PS), with the most common subtypes of DORV being as follows:
(i) Tetralogy of Fallot-type variant (subaortic VSD with PS).
(ii) Transposition of great arteries-type variant (sub-pulmonary VSD without PS): Taussig-Bing anomaly.
(iii) VSD-type variant (subaortic VSD without PS).
(iv) Univentricular heart–type variant (DORV with mitral atresia, unbalanced atrioventricular canal, or presence of severe hypoplasia of one of the ventricles).
ICD code
Q20.1
Incidence
DORV is a rare anomaly, representing about 1% of congenital heart defects, and has an incidence of approximately 0.09 per 1,000 live births. It is found equally in males and females.
Similar to other congenital heart diseases, DORV is more common in fetal series and has been reported in up to 6% of prenatally diagnosed congenital heart disease. Due to its association with other major cardiac anomalies, extracardiac anomalies, and chromosomal or non-chromosomal genetic syndromes, the incidence of fetal demise is elevated and can be the reason for higher prenatal prevalence (reported to be 0.46%).
Pathogenesis
In early normal development, the primitive heart develops from two tubes that merge into one. This tube quickly forms five distinct regions which include the truncus arteriosus in the cranial portion. In normal cardiac development, the cardiac tube is twisted and turns on itself in a rightward direction (dextral looping), and the truncus arteriosus shifts toward the midline. A septum grows along this truncus, in a spiral way, to form 2 vessels- the aorta and the pulmonary artery. The spiral septum leads the aorta to travel posteriorly and to the right of the pulmonary artery.
Embryologic pathogenesis of DORV is thought to be related to either abnormal cono-truncal rotation or a failure of the leftward shift of the aortic/pulmonary conus.
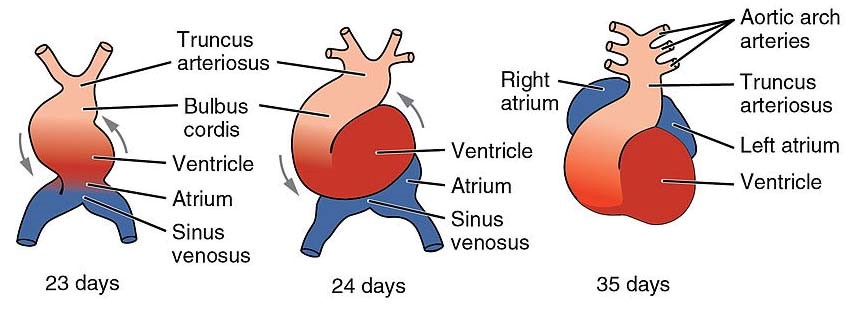
Etiology
The prevalence of DORV was found to be increased in diabetic pregnancies, with odds ratio of 21.3 2
Pathology and associated anomalies
DORV is frequently associated with additional major cardiac and extracardiac anomalies with an incidence of up to 90% in pre- and postnatal series. 3–8
The most common cardiac anomalies described are right ventricular outflow tract obstruction (RVOTO) and LVOTO. Other anomalies associated with DORV are hypoplastic left ventricle (HLV) with or without mitral valve atresia, atrial septal defect, atrioventricular septal defect, aortic coarctation, right aortic arch, persistent left superior vena cava and anomalous pulmonary venous return.
DORV can be part of isomerism 9.
Extracardiac anomalies are common and can be found in up to 60% of the cases. The extracardiac anomalies are not specific, and can include skeletal, cerebral, renal, and facial anomalies.
Chromosomal and syndromic anomalies are reported in 14-41% of DORV cases3–5,10. This includes aneuploidy (most commonly Trisomy 13 and 18) and 22q11.2 microdeletion syndrome.
Recurrence risk
For isolated DORV, the risk for recurrence is approximately 1.5% to 2%.
If aneuploidy is present (e.g., trisomy 18), the recurrence risk is low.
Fetal Diagnosis
Gray-scale – The four-chamber view in DORV will often appear normal in the first and second trimesters as the ventricles and atrioventricular valves are usually of normal size, The VSD may not be readily visible on a 4-chamber view. The five-chamber view / outflow tract views are abnormal, demonstrating abnormal origins of the outflow tracts. Both great arteries arise from the morphological RV. Lack of the normal fibrous continuity of the mitral valve and aortic valve may be noted. The location of the VSD relative to the outflow tracts must be noted by careful sweeps from the 4-chamber view to the outflow tract view. The anatomic relationship of the great arteries can be assessed by the 3-vessel view and 3 vessel tracheal view,
When the diagnosis is confirmed, the following associated cardiac anomalies should be assessed and ruled out:
Outflow tract obstruction
Mitral valve abnormalities (atresia, stenosis, straddling)
AV septal defects
Aortic coarctation or interruption of aorta
Features of heterotaxy syndrome including dextrocardia, systemic venous and pulmonary venous abnormalities
Colour Doppler – Colour flow is helpful to demonstrate presence of shunting across the VSD (usually unidirectional from the LV to the RV). The presence or absence of forward flow across both outflow tracts is demonstrated. Outflow tract stenosis can be identified by turbulent higher velocity flow with colour aliasing. Valvular regurgitation can be demonstrated with reversed colour flow. The direction of flow at the level of the ductus arteriosus can be identified, with reversal of this flow (from aorta to pulmonary artery) indicating severe pulmonary stenosis or atresia. Demonstration of colour flow in the aortic arch and assessment of its direction of flow can identify severe aortic valve stenosis or aortic arch obstruction (retrograde flow in the aortic arch).
Three-dimensional ultrasound and cardiac MRI/CT are useful in the postnatal period to further define anatomy and spatial relationships. However, there is limited utility of these investigations at present in fetal life.11,12.
Differential diagnosis
1. Tetralogy of Fallot (TOF) – the ventriculo-arterial connections are concordant, and the great vessels are not malposed. The aorta is overriding the ventricular septum, with more than 50% origin from the left ventricle, there is mitral valve to aortic valve continuity, and a degree of right ventricular outflow tract obstruction.
2. Transposition of the great arteries (D-TGA) – outflow tracts are parallel, with abnormal ventriculo-arterial connection (aorta arises from morphologic RV, and pulmonary artery arises from morphologic LV).
3. Truncus arteriosus (also named common arterial trunk) – is part of the differential diagnosis when the congenital heart
Implications for sonographic diagnosis
Sonographic diagnosis is important as DORV lesions often require neonatal intervention. The presence of outflow tract obstruction often necessitates initiation of Prostaglandin E following delivery to maintain ductal patency. Some types of DORV, such as sub-pulmonary VSD type, may present with physiology similar to complete transposition of the great arteries and profound cyanosis. The correct diagnosis will allow delivery in an appropriate centre and initiation of neonatal therapy.
Implications for sonographic screening
DORV is a complex congenital heart anomaly, however it is diagnosable using standard screening cardiac views. The four-chamber view will often demonstrate an abnormality such as a large VSD or ventricular size disproportion. However, it is the outflow tract views, including 3 vessel view, that will demonstrate the abnormal anatomy. Parallel outflow tracts, abnormal position of the great arteries, or size discrepancy between the great arteries can all be identified by these views. These abnormal screening views can then prompt referral to a specialist in fetal cardiology to secure the complete diagnosis.
Prognosis
The intrauterine course is usually uneventful unless associated with chromosomal abnormalities or other severe heart anomalies which can lead to cardiac failure, hydrops, and in utero demise.
Recent data suggests significant improvement in survival, with a survival rate of 80-90% after surgical repair. 13.
Prenatal Management
• Fetal echocardiography - should be performed to confirm the diagnosis and assess for additional cardiac malformations.
• Genetic counseling and testing should be offered.
• Prenatal consultation with neonatology and pediatric cardiology is recommended to ensure timely and appropriate treatment during the newborn period.
• Serial sonographic assessment is advised to confirm appropriate fetal growth and absence of hydrops.
• Delivery should occur in a tertiary care center, with availability of neonatal cardiac support such as interventional cardiology and cardiac surgery.
References
1. Abuhamad A, Chaoui R. A Practical Guide to Fetal Echocardiography Normal and Abnormal Hearts. Third edition. Lippincott Williams & Wilkins, a Wolters Kluwer business; 2016.
2. Ferencz C, Rubin JD, McCarter RJ, Clark EB. Maternal diabetes and cardiovascular malformations: Predominance of double outlet right ventricle and truncus arteriosus. Teratology. 1990;41(3). doi:10.1002/tera.1420410309
3. Gottschalk I, Abel JS, Menzel T, Herberg U, Breuer J, Gembruch U, Geipel A, Brockmeier K, Berg C, Strizek B. Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center. Journal of Perinatal Medicine. 2019;47(3):354-364. doi:10.1515/jpm-2018-0316
4. Obler D, Juraszek AL, Smoot LB, Natowicz MR. Double outlet right ventricle: Aetiologies and associations. Journal of Medical Genetics. 2008;45(8):481-497. doi:10.1136/jmg.2008.057984
5. Gedikbasi A, Oztarhan K, Gul A, Sargin A, Ceylan Y. Diagnosis and prognosis in double-outlet right ventricle. American Journal of Perinatology. 2008;25(7). doi:10.1055/s-0028-1083840
6. Chaoui R, Körner H, Bommer C, Göldner B, Bierlich A, Bollmann R. [Prenatal diagnosis of heart defects and associated chromosomal aberrations]. Ultraschall Med. 1999;20(5).
7. Sivanandam S, Glickstein JS, Printz BF, Allan LD, Altmann K, Solowiejczyk DE, Simpson L, Perez-Delboy A, Kleinman CS. Prenatal diagnosis of conotruncal malformations: Diagnostic accuracy, outcome, chromosomal abnormalities, and extracardiac anomalies. American Journal of Perinatology. 2006;23(4):241-245. doi:10.1055/s-2006-939535
8. Allan LD, Sharland GK, Milburn A, Lockhart SM. PEDIATRIC CARDIOLOGY Iagnosis Of 1,006 Consecutive Cases of Congenital Heart Disease in the Fetus.
9. Berg C, Geipel A, Kamil D, Krapp M, Breuer J, Baschat AA, Knöpfle C, Germer U, Hansmann M, Gembruch U. The syndrome of right isomerism - Prenatal diagnosis and outcome. Ultraschall in der Medizin. 2006;27(3). doi:10.1055/s-2005-858639
10. Hartge DR, Niemeyer L, Axt-Fliedner R, Krapp M, Gembruch U, Germer U, Weichert J. Prenatal detection and postnatal management of double outlet right ventricle (DORV) in 21 singleton pregnancies. In: Journal of Maternal-Fetal and Neonatal Medicine. Vol 25. ; 2012:58-63. doi:10.3109/14767058.2011.561387
11. Bharucha T, Hlavacek AM, Spicer DE, Theocharis P, Anderson RH. How should we diagnose and differentiate hearts with double-outlet right ventricle? Cardiology in the Young. 2017;27(1):1-15. doi:10.1017/S1047951116001190
12. Priya S, Nagpal P, Sharma A, Pandey NN, Jagia P. Imaging Spectrum of Double-Outlet Right Ventricle on Multislice Computed Tomography. Journal of Thoracic Imaging. 2019;34(5):W89-W99. doi:10.1097/RTI.0000000000000396
13. Oladunjoye O, Piekarski B, Baird C, Banka P, Marx G, del Nido PJ, Emani SM. Repair of double outlet right ventricle: Midterm outcomes. In: Journal of Thoracic and Cardiovascular Surgery. Vol 159. Mosby Inc.; 2020:254-264. doi:10.1016/j.jtcvs.2019.06.120
This article should be cited as: Willner I, Lougheed J: Double Outlet Right Ventricle, Visual Encyclopedia of Ultrasound in Obstetrics and Gynecology, www.isuog.org, January 2023.
Leave feedback or submit an image
We rely on your feedback to update and improve VISUOG. Please use the form below to submit any comments or feedback you have on this chapter.
If you have any images that you think would make a good addition to this chapter, please also submit them below - you will be fully credited for all images used.
Feedback form
Please note that the maximum upload size is 5MB, and larger images and video clips can be sent to [email protected].
